Issue 4
13 January 2021
The newsletter on COVID-19-vaccination is an RIVM publication with up-to-date information for professionals involved in COVID-19 vaccination.
Approval for second vaccine against COVID-19
On Wednesday 6 January 2021, the European Medicines Agency (EMA) issued a positive scientific recommendation for the second COVID-19 vaccine in the European Union: the mRNA vaccine developed by Moderna. That same day, the European Commission announced that it had approved the vaccine for the European market. Meanwhile, the ‘Summary of Product Characteristics’ is also available, as well as a Dutch factsheet on the vaccine.
Updated implementation guidelines and e-learning module on COVID-19 vaccination
In response to the availability of the Modern vaccine, the implementation guidelines for COVID-19 vaccination in 2021 and the e-learning module on COVID-19 vaccination have been updated. Section 1.3 of the implementation guidelines provides a summary of the key changes compared to the previous version.
Please note: adverse reactions can be reported to Pharmacovigilance Centre Lareb using the online reporting form (only available in Dutch) at www.lareb.nl, www.mijnbijwerking.nl https://meldformulier.lareb.nl/forms/covid.
Health Council advisory reports and ministerial decisions regarding vaccination strategy
Partnership between OMT and Health Council
On 4 January 2021, the first consultation took place between the Outbreak Management Team (OMT) and the Health Council of the Netherlands (Gezondheidsraad) to arrive at joint advisory opinions regarding the COVID-19 vaccination strategy and optimal alignment with the efforts to control the COVID-19 epidemic.
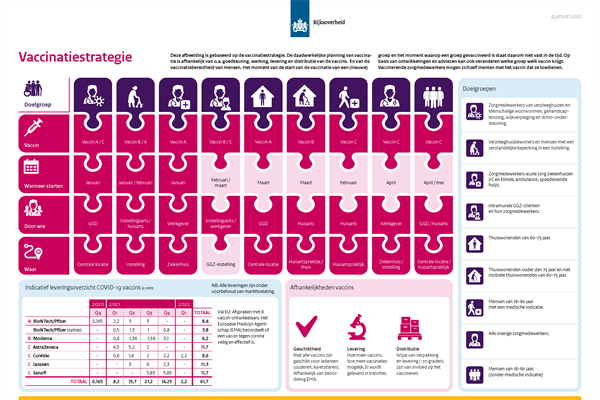
The OMT and the Health Council advise inviting all people over 60 years old (starting with older people aged 75 and up) for vaccination by the Municipal Public Health Services (GGDs); those who are truly unable to come to the GGD vaccination site would then receive a new invitation for vaccination from their GP. Since the most significant health benefits can be achieved in the priority target group of adults aged 60 and up, they advise allocating at least 90% of the mRNA vaccines made by BioNTech/Pfizer and Moderna for vaccinating this group, and no more than 10% for professional groups.
The Minister endorses the joint advisory opinion from the OMT and the Health Council to deploy the available vaccines for the older target group as much as possible, and to make use of every possible option to have them come to the GGD vaccination site.
Moderna vaccine
On 11 January 2021, the Health Council of the Netherlands published its advisory report (currently only available in Dutch) on the second mRNA vaccine approved in Europe against COVID-19, developed by Moderna, and its suitability for the specific target groups. Similar to the first vaccine, the Health Council advises primarily using this vaccine for older people aged 60 and up, starting with the oldest, regardless of whether they are in a medical risk group. This is because the vaccine has been very efficacious in older people, and the burden of disease resulting from COVID-19 is highest in this group.
The Minister will be following the advisory opinion of the Health Council: the BioNTech/Pfizer and Moderna vaccines currently available will be used primarily for the group aged 60+. Vaccinations will still be completed for the first group of healthcare workers. Vaccination will start sooner for the following groups:
- Residents of nursing homes and institutions for people with intellectual disabilities whose treating physician is a geriatrician or physician for people with intellectual disabilities (not a GP), and who have a medical file in the institution (vaccine: BioNTech/Pfizer, from 18 January 2021);
- Residents of small-scale residential facilities and institutions for people with a disability whose GP has medical responsibility for their care (vaccine: Moderna, from 25 January 2021);
- People aged 60 years and up who are living at home (vaccine: wherever possible, BioNTech/Pfizer for people who can come to the GGD vaccination site and Moderna for people who are not mobile, and if possible also AstraZeneca, starting in mid-February 2021).
The flowchart and roadmap (in Dutch) reflect the implementation of the vaccination strategy as it is currently planned.
Upcoming events
Extra attention will be focused on COVID-19 vaccination in various ways in the next few weeks. (Please note: all these events will be exclusively in Dutch.)
- Wednesday 13 January 2021, 20:00-21:00 hrs: webinar for general practitioners (MedischeScholing.nl);
- Thursday 14 January 2021, 19:00-21:00 hrs: looking back webinar for doctors and medical students (KNMG);
- Thursday 21 January 2021, 20:00 hrs: Facebook Live session “A safe vaccine in record time, how is that possible? Ask science!” (the National Science Agenda).