No. 1, 3 December 2020
3 December 2020
The newsletter on COVID-19-vaccination is an RIVM publication with up-to-date information for professionals involved in COVID-19 vaccination.
Launch of the newsletter
As of today, RIVM will start publishing the newsletter on COVID-19-vaccination. This newsletter will regularly inform you – the professionals involved in the implementation and other interested professionals – about the latest updates regarding COVID-19 vaccination.
Current situation regarding COVID-19 vaccination
Watch this video for an update on the current situation (in Dutch) regarding COVID-19 vaccination. The video was compiled from an RIVM press conference on Wednesday 25 November 2020.
Dutch government aligns with Health Council advisory report
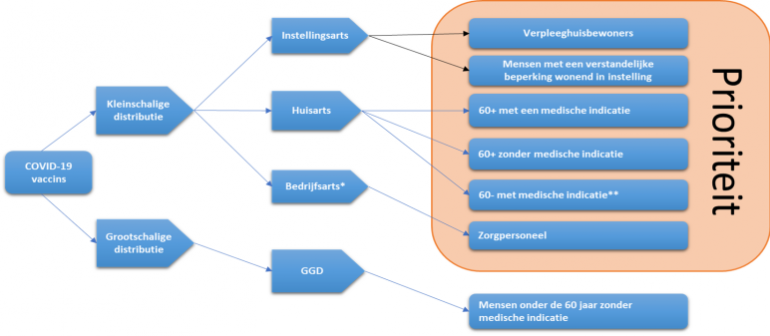
On 20 November 2020, the Government response to the advisory report of the Health Council of the Netherlands was announced: the government will be adopting the recommendation to start by vaccinating the elderly and sick against COVID-19. The recommendations set out in Health Council of the Netherlands' advisory report are in line with the objectives formulated by the Dutch government to control the virus: to protect vulnerable people and prevent the healthcare system from becoming more overburdened.
The first vaccines will be made available to nursing home residents and all people with intellectual disabilities living in an institution. Vaccination will also be offered to all employees of these nursing homes and institutions. This will be followed by other groups, such as:
- People aged 60 and above who have a medical indication (starting with the oldest age groups)
- People aged 60 and above who do not have a medical indication (starting with the oldest age groups)
- People under the age of 60 who do have a medical indication
- Care workers who work with these groups
- and the care workers who are in direct contact with patients who have COVID-19.
Finally, vaccination will be offered to other employees working in healthcare, and to people under the age of 60 who do not have a medical indication. The final vaccination strategy will be determined by the suitability of vaccines for specific target groups, the quantity of vaccines available, and the way in which the vaccines are delivered.
* Or as defined by other qualified personnel in hospitals, for example.
** If people from this target group cannot be vaccinated, then informal carers and care workers who pose a risk to them are eligible, as well as care workers who have direct contact with patients.
Candidate vaccines against SARS-CoV-2
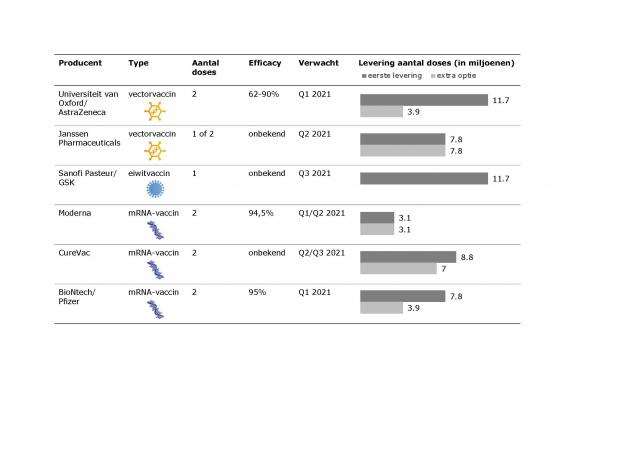
Various types of SARS-CoV-2 vaccines are being developed. The Netherlands has reached agreements on six vaccine candidates so far (see table). By now, contracts have also been signed with the manufacturers of all these candidate vaccines. Negotiations on contracts for Novavax, a seventh candidate vaccine, are ongoing.
The European Medicines Agency (EMA) is currently reviewing the marketing authorisation applications for the mRNA vaccines made by BioNtech/Pfizer and Moderna. As it stands now, the EMA Committee for Medicinal Products for Human Use (CHMP) will discuss the BioNtech/Pfizer vaccine on 29 December 2020 and the Moderna vaccine on 12 January 2021. If the data meet the authorisation requirements, a decision will follow.
Table: Overview of candidate vaccines
Communication
Public
The general public can find information about the vaccine at government.nl. The people who will be invited to be vaccinated will receive a letter and an infographic containing the most important information about the vaccination and a link to the website. In addition, a public campaign is being developed to inform people and encourage them to get vaccinated. The campaign will launch this month, starting with radio commercials, among other things. These are mainly intended to inform people and refer them to the website. More media outlets will be included in the campaign later, such as TV commercials.
Professionals
Information for professionals involved in implementation of the COVID-19 vaccination is provided at COVID-19 vaccination - for professionals. The video compilation of the press conference (in Dutch) can also be viewed. As soon as it becomes available, the page will be supplemented to include guidelines, the e-learning module, Q&As, information about the vaccines, patient information leaflets, etc. A special telephone number will also be provided for medical questions and questions about the process.
Information editors
Editors: Vaccination implementation, National Coordination Centre for Communicable Diseases Control (LCI)
For questions and/or comments about this newsletter, please send an e-mail.