Issue 3, 6 January 2021
6 January 2021
The newsletter on COVID-19-vaccination is an RIVM publication with up-to-date information for professionals involved in COVID-19 vaccination.
COVID-19 vaccination campaign starts
The first COVID-19 vaccination in the Netherlands was administered on Wednesday 6 January at the GGD vaccination site in Veghel. Vaccination has also started in Dutch hospitals. The GGD vaccination sites in Rotterdam and Houten are open as of Friday 8 January, and the GGD vaccination sites in Amsterdam, Assen and The Hague will be opening from Monday 11 January on. All 25 GGD vaccination sites will be open for COVID-19 vaccinations as of 15 January 2021.
Frequently asked questions about COVID-19 vaccination
An FAQ about COVID-19 vaccination is now available for healthcare professionals. The public website at coronavaccinatie.nl and the website for professionals link to these questions.
Logistical and medical questions about COVID-19 vaccination
From 4 January 2021 on, the special telephone number 088-6788900 will be available from 8:00-20:00 hrs for
- logistical questions (questions about vaccines, vaccine delivery, vaccine management, product complaints and vaccine incidents,
- medical questions (first check the COVID-19 Vaccination Guidelines and the Q&As) and
- registration questions (requesting vaccination data, if recorded in the central register known as CIMS).
This number is intended for the medical professionals who are administering the vaccinations. Healthcare workers and others who have questions about their own vaccination can be referred to the Q&As, the website at www.coronavaccinatie.nl (in Dutch) or to the organisation that administers the vaccination.
Medical questions submitted after 20:00 hrs that cannot wait until the next day are transferred to backup support. Less urgent medical questions can also be emailed to the National Coordination Centre for Communicable Disease Control (LCI) at LCI.voorwacht@rivm.nl.
Adverse reactions (side-effects) of the vaccination can be reported to Lareb on telephone number 073-6469700 or at www.lareb.nl.
Implementation Guidelines for COVID-19 Vaccination in 2021
These implementation guidelines for COVID-19 vaccination 2021 (version 1.0) were drafted by RIVM together with the relevant (implementing) professional groups and organisations and finalised on 31 December 2020. This version includes a section outlining the changes compared to the earlier draft version 0.4 (24 December 2020).
E-learning module on COVID-19 vaccination
The e-learning module on COVID-19 vaccination is now available in Dutch and includes a general section (covering such topics as vaccine development, target groups, stakeholder roles, implementation aspects and dialogue scripts) as well as vaccine-specific chapters. The application for accreditation (2 credits) has been submitted to: ABFE, ABC1, ABSG, V&VN and Verpleegkundig specialisten.
Public campaign on COVID-19 vaccination
The public campaign was launched in December, featuring on advertisements and social media. A TV commercial has now also be released: COVID-19 vaccination: “We are rolling up our sleeves” - YouTube.
Advisory report of the Health Council of the Netherlands
On 24 December 2020, the Health Council of the Netherlands published its advisory report on Comirnaty (the first mRNA vaccine approved in Europe against COVID-19, developed by Pfizer and BioNTech) and its suitability for the specific target groups. The Health Council advises primarily using this vaccine for older people aged 60 and up, starting with the oldest. The vaccine works even better than expected in this group, and they also have the highest risk of severe illness and death due to COVID-19.
Ministerial decree on vaccination strategy
The vaccination strategy is primarily focused on reducing serious illness and deaths as a result of COVID-19. The following three developments have led the Cabinet to make additional choices regarding implementation of the vaccination strategy:
- The advisory opinion of the Health Council of the Netherlands to use the Comirnaty vaccine (BioNTech/Pfizer) as much as possible in people aged 60 years and older, starting with the oldest.
- The conclusion reached by RIVM that, after reconsideration, the Comirnaty vaccine is logistically unsuitable for use with vulnerable groups in GP practices.
- The changing epidemiological situation, including the developments regarding the UK variant of the virus (which seems to be spreading more rapidly) and the increased pressure in acute COVID-19 care in hospitals.
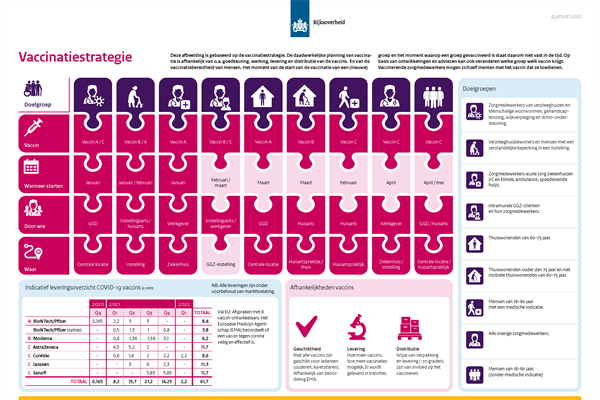
The following flowchart (in Dutch) and roadmap (in Dutch) reflect the implementation of the vaccination strategy as it is currently planned.
Upcoming events
Extra attention will be focused on COVID-19 vaccination in various ways in the next few weeks.
(Please note: all these events will be exclusively in Dutch)
- Wednesday 13 January 2021, 20:00-21:00 hrs: webinar for general practitioners (MedischeScholing.nl);
- Thursday 14 January 2021, 19:00-21:00 hrs: webinar for doctors and medical students (KNMG);
- Thursday 21 January 2021, 20:00 hrs: Facebook Live session “A safe vaccine in record time, how is that possible? Ask science!” (the National Science Agenda).