colophon
Issue 26, 22 July 2021
The newsletter on COVID-19 vaccination is an RIVM publication with up-to-date information for professionals involved in COVID-19 vaccination.
Progress report on the COVID-19 vaccination campaign
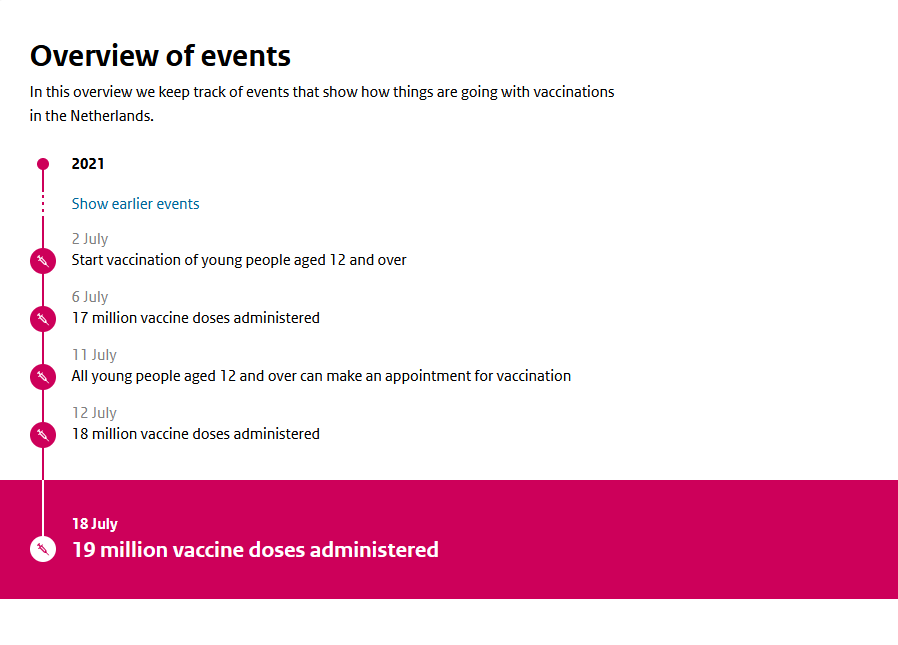
The 19 millionth COVID-19 vaccination was administered this week.
European Medicines Agency (EMA)
|
The EMA has issued a direct healthcare professional communication (DHPC) for the Janssen vaccine (addition of capillary leak syndrome and update on ‘thrombosis with thrombocytopenia syndrome’, TTS) and the mRNA vaccines Comirnaty© and Spikevax© (risk of myocarditis and pericarditis). |
Update on shorter interval between first and second vaccination
Regarding the shorter interval between appointments to receive an mRNA vaccine (the COVID-19 vaccines made by Pfizer/BioNTech and Moderna) from the Municipal Public Health Services (GGDs), GGD GHOR Netherlands has announced that an interval of 28 days will be used from Monday 19 July 2021 on. This is the minimum permissible interval for the Moderna vaccine according to the package leaflet, and it is not possible to make a distinction between the Pfizer/BioNTech and Moderna vaccines when scheduling vaccinations. Since young people under 18 years old are only vaccinated with the Pfizer/BioNTech vaccine, it is possible to reduce the interval for this group even further, down to 21 days. An interval of 35 days had been used so far. It will take some time to arrange this properly in the current systems, so for the time being the shorter interval only applies to new appointments. There is an exception for young people under 18: they can bring forward an existing appointment for the second vaccination if they want to.
Leftover COVID-19 vaccines
|
ow that more and more people have had a second vaccination, general practitioners, institutions, hospitals or other implementing organisations may still have leftover COVID-19 vaccines (in unopened vials). With the help of the RIVM vaccine brokers, options are being explored to see whether the vaccines can be used elsewhere, for example in another, similar organisation or for another target group. If you have unexpired AstraZeneca vaccines in stock, you can notify the LCC support team by telephone. If you have any other vaccine brands that are not yet expired, please submit a notification by email to vaccinmakelaar@rivm.nl. Any remaining open vials or vaccines that are past their expiry date are required by law to be destroyed according to the usual procedures. RIVM has drawn up instructions for this (only available in Dutch). Unused vaccines only need to be reported to the LCC support team or vaccine broker once, during the period before the expiry date. If the vaccine passes its expiry date, you do not need to report that separately. |
Children and adolescents aged 12-17 are willing to be vaccinated
The majority of this age group (72%) want to be vaccinated; those aged 16-17 years are slightly more willing to be vaccinated (79%) than those aged 12-15 years (68%). These findings are from research conducted by the Corona Behavioural Unit at RIVM. More information is available here (in Dutch).
Public communication
Injection anxiety |
|
Anxious about getting an injection? 6 tips to overcome a fear of injections. |
Combined vaccinations |
|
In this video (in Dutch), Lieke Sanders, Professor of Immunology and Infectious Diseases, answers 4 questions about combined vaccination: how safe is it to combine vaccines and how effective is the protection offered by the combination of different vaccines? |
editors
Editors: Vaccination implementation, National Coordination Centre for Communicable Diseases Control (LCI).
For questions and/or comments about this newsletter, healthcare professionals can send a message to vaccin-covid@rivm.nl.
Private citizens can call the public information number 0800 - 1351 with their questions.