colofon
1 March 2021
The newsletter on COVID-19-vaccination is an RIVM publication with up-to-date information for professionals involved in COVID-19 vaccination.
Progress report on the COVID-19 vaccination campaign
Vaccination against COVID-19 is accelerating steadily. The millionth COVID-19 vaccination in the Netherlands was administered just over a week ago.
From last week on, vaccination has now also started for mental health clients in inpatient or residential care in institutions. Both clients and care workers in those institutions will be vaccinated with the AstraZeneca vaccine. People aged 65 and older will receive the Moderna vaccine as soon as it is available.
As of last Thursday, care workers in district nursing who have direct contact with clients can also make an appointment for a COVID-19 vaccination. They will receive the AstraZeneca vaccine. People aged 65 and older and people who have specific medical conditions will receive the Pfizer/BioNTech vaccine.
Vaccination prioritisation for high-risk groups
The recent letter to Parliament greenlighted earlier vaccination of people at very serious risk of severe illness or death due to COVID-19 infection. A vaccination route has now been set in motion for two risk groups: people with Down’s Syndrome and people with morbid obesity. They will be invited and vaccinated by their GPs. The vaccination route for other groups who are being treated by certain medical specialists is currently being developed. The latest information is available here.
Good to know:
Everyone who qualifies for this route will automatically receive an invitation. It is not necessary to contact a doctor about this.
It may happen that people in the high-risk group will receive an invitation for vaccination on the basis of their age before receiving an invitation via the medical specialist. Given the current limited availability of vaccines, the advice is always to make use of the first vaccination opportunity offered, and not to wait to be vaccinated. All registered vaccines offer protection against (severe) COVID-19.
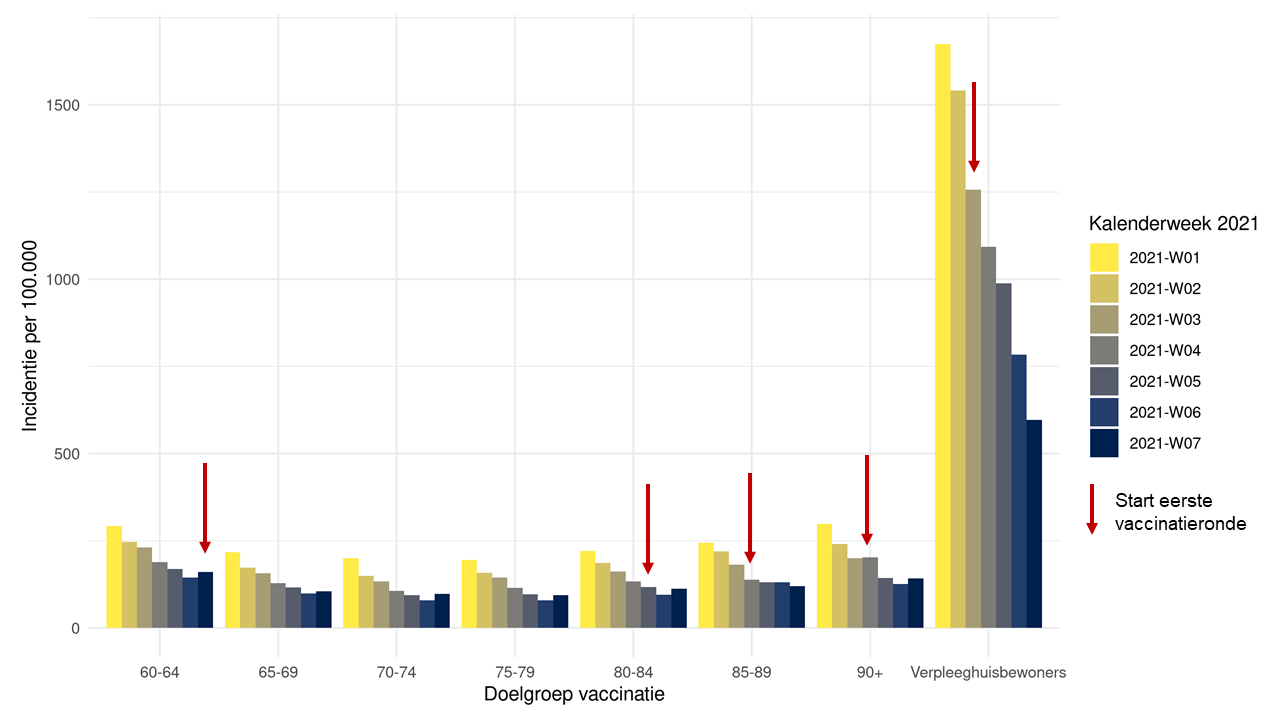
Impact of COVID-19 vaccination
The number of infections among nursing home residents is decreasing in the Netherlands. On the other hand, infections among older people living at home have been on the rise again compared to two weeks ago. The continued significant decrease among nursing home residents indicates that vaccination is having a beneficial effect against the coronavirus.
Side-effects of COVID-19 vaccination and impact on employee absences
As indicated in the package leaflets, side-effects such as fever, headache and fatigue occur after vaccination with COVID-19 vaccines in a significant proportion of those who are vaccinated. Side-effects such as muscle pain, joint pain, chills, nausea and vomiting may also occur after vaccination with Comirnaty (Pfizer/BioNTech), Moderna and AstraZeneca vaccines. These reactions to vaccination are harmless, and are the result of the immune system responding to the vaccine. Paracetamol (normal dosage) may offer relief. Most symptoms resolve spontaneously within 1-2 days. However, the side-effects can be inconvenient, and may cause some people to miss work in the first 24-48 hours after vaccination.
When COVID-19 vaccinations are scheduled for certain professional groups/departments/teams, such as employees working in the healthcare sector – but also other groups – this effect of vaccination should be taken into account for reasons of continuity. This applies to both the first and the second vaccination. It may therefore be important to stagger the timing of vaccination appointments.
Symptoms are more pronounced in young people than in older people. After the second vaccination, these side-effects increase (in number and severity) in response to the mRNA vaccines (Comirnaty and Moderna). The AstraZeneca vaccine can also cause similar side-effects after the first and second vaccinations.
Updated implementation guidelines for COVID-19 vaccination
The implementation guidelines for COVID-19 vaccination 2021 have been updated. The latest version of the implementation guidelines is always available online (in Dutch). Section 1.3 outlines the main changes compared to the previous version. This includes new guidance on assessing allergic reactions following COVID-19 vaccination and an updated Handbook on COVID-19 vaccination for immunocompromised patients.
Public communication
The video ‘From invitation to registration’ (in Dutch) shows the steps you take from receiving the invitation to picking up your registration card.
Upcoming events
Extra attention will be focused on COVID-19 vaccination in various ways in the next few weeks. (Please note: all these events will be exclusively in Dutch.)
- Thursday 4 March, 19:30-21:00: webinar on COVID-19 vaccination campaign: good vaccine management for vaccine administrators responsible for COVID-19 vaccines and managers and implementers of organisations responsible for COVID-19 vaccination (RIVM in cooperation with the Health and Youth Care Inspectorate (IGJ)); click here to sign up.
- Friday 5 March, 13:30-16:30: training session on ‘vaccination & behaviour’ for communication professionals from municipalities, GGDs and security regions (RIVM Corona Behavioural Unit), registration via coronagedragsunit@rivm.nl (there are still a few places available).
editors
Editors: Vaccination implementation, National Coordination Centre for Communicable Diseases Control (LCI).
For specific questions about the implementation guidelines for COVID-19 vaccination, healthcare professionals can contact the LCI (Lci.voorwacht@rivm.nl).
Private citizens can call the public information number 0800 - 1351 with their questions.
For questions and/or comments about this newsletter, please send an e-mail.