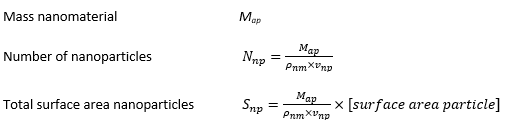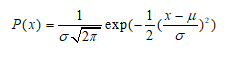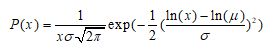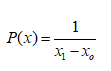ConsExpo nano is a web based tool that can be used to estimate inhalation exposure to nanomaterials in consumer sprays. The tool adapts the ConsExpo spray tool for regular substances and combines this model with the ICRP deposition and clearance model to estimate inhaled and deposited doses. This page provides background on the models implemented in the ConsExpo nano tool.
The use of nanomaterials in spray products may lead to inhalation exposure of consumers to the nanomaterial. Of special concern is the potential deposition of nanomaterial in the pulmonary (alveolar) region. Poorly soluble material is only slowly cleared from this region. Alveolar deposition may lead to protracted local exposure and accumulation of the nanomaterial in the case of frequent exposure. The duration and magnitude of alveolar load of a nanomaterial has been identified as one of the primary determinants of local adverse effects such as inflammation in the lung (Braakhuis et al., (2014)).
ConsExpo nano is a tool that can be used to estimate inhalation exposure as the inhaled dose and the alveolar load of nanomaterials for consumers using spray products. The tool combines models for exposure, deposition and clearance to estimate alveolar load. The exposure is expressed in different dose metrics for quantitative risk assessment.
For comparison of the exposure evaluation in ConsExpo nano with a hazard study in rat, the latter can also be simulated in ConsExpo nano, to allow for a detailed comparison of exposure levels with hazard data.
During use of a spray product, the nanomaterial is released and may become airborne.
Nanomaterials will generally be released as part of aerosol particles. Aerosol particles carrying the nanomaterial may be inhaled and may deposit in various regions of the respiratory tract, in particular in the alveolar region. Deposited particulate material will be removed from the alveoli by clearance (e.g. macrophage and ciliary clearance), dissolution and interstitialisation. For poorly soluble nanomaterials, all these processes are expected to be slow.
The total alveolar load of a nanomaterial at any given time is the result of competing kinetic processes of (repeated) exposure, deposition and clearance.
The magnitude of the aerosol air concentration is determined by the spray and the conditions of its use. It is in particular a result of the mass generation rate of the spray, the size (distribution) and mass density of the generated aerosol, the duration of use of the spray, ventilation of the air et cetera.
Deposition of the inhaled aerosol in the respiratory tract will depend on both aerosol characteristics and human physiology. The most important aerosol characteristics that determine deposition are the aerosol diameter and its mass density. The critical aspects of human physiology are the geometry of the lung and the breathing pattern (i.e. intensity of breathing) of the exposed person.
To model exposure and the alveolar load, ConsExpo nano combines a number of models.
Alveolar load of an inhaled nanomaterial is the result of three distinct processes:
- Inhalation of aerosol particles containing the nanomaterial that have become airborne after the use of a spray product
- Deposition of the inhaled aerosol particles in the respiratory tract
- Clearance of the inhaled material from the alveoli by the pulmonary macrophage system and by dissolution of nanomaterial in the macrophages after phagocytosis
ConsExpo nano links four separate models to estimate time dependent alveolar load:
- A model to estimate the concentration and the inhaled mass of the sprayed aerosol
- A model to estimate the deposition in the alveoli based on the aerosol diameter and mass density
- A model to simulate clearance of the material from the alveoli, assuming non-soluble particle load in the alveoli
- A kinetic model that accounts for dissolution of the material using user-specified information on the dissolution rate of the material in the alveoli (e.g. in the macrophage or the lung lining fluid)
For the estimation of the inhalation exposure, two options are available to the user. First, the user may use the ‘spray scenario’. With this option, the tool uses the a model that simulates the external aerosol concentration in indoor air that is equivalent to the ConsExpo ‘exposure to spray model’. A description of this model and the equations can be found in Delmaar et al., (2005). Alternatively, the user may specify a ‘custom scenario’. In this case the user gives the air concentration, aerosol particle size and exposure duration, from which inhalation exposure is estimated. This is done by assuming that the air concentration Cair is completely inhalable and thus the inhaled amount Ainh is determined as:
Ainh= Qinh×Cair×T
Where Qinh is the inhalation rate (volume per time) and T the exposure duration.
The models used to simulate deposition and clearance from the alveoli is an implementation of the ICRP deposition model. ICRP (1994) and ICRP (2015) give model equations and values for the model parameters derived from inhalation and deposition experiments. The reference provides different parameter sets depending on age, gender and activity level. ConsExpo nano implements two models: the model for males and females performing light exercise.
Dissolving of the nanomaterial is modelled by a first order kinetic process in which the user specifies a dissolution rate of the nanomaterial per day. Only relatively low dissolution rates will be accepted as input as the deposition and clearance models have been developed for poorly soluble aerosols.
In summary, ConsExpo nano estimates inhaled dose and alveolar load of a nanomaterial in spray particles. The following assumptions are made:
- The alveolar load varies in time and depends on the inhalation, deposition and clearance of particulate matter.
- The nanomaterial is released as part of an aerosol. The nanomaterial is transported in the aerosol particles in indoor air and through the respiratory tract. I.e. the properties of the aerosol particle will ultimately determine inhalation and deposition of the nanomaterial.
- The aerosol particles are assumed to consist of the nanomaterial only. No other components are assumed to be present.
- The aerosol particles are assumed to remain unaltered in the process of inhalation and deposition. Only when deposited in the alveolar region, changes in aerosol due to dissolution (in lung lining fluid or alveolar macrophages) are considered.
- Dissolution of the nanomaterial in the alveoli (either in macrophage or lung lining fluid) is considered as a first order kinetic process, characterised by a single, constant dissolution rate. This rate is to be specified by the user.
In the risk assessment for regular (i.e. non-nano material) substances, inhalation exposure is usually expressed on the basis of the inhaled mass. The safety of the use of the substance is assessed by comparing mass-based exposure estimates to levels of inhaled mass that are believed not to lead to adverse health effects.
For nanomaterials, it has been shown that similar mass doses of particles with different geometry may induce very different levels of effect in test animals. Therefore, mass does not seem to be an appropriate metric to base the risk characterisation on.
Different alternative dose measures have been suggested in literature, but consensus on a common metric, or whether such a metric is feasible at all, has not been achieved.
ConsExpo nano expresses the alveolar load in different, potentially relevant dose metrics.
The following dose metrics are included:
- the number of nanoparticles
- the total surface area of nanoparticles (mm2)
- the total mass of nanoparticles (mg)
- the total volume of the nanoparticles (mm3)
- the number of aerosol particles
- the total surface area of aerosol particles (mm2)
- the total volume of the aerosol particles (mm3)
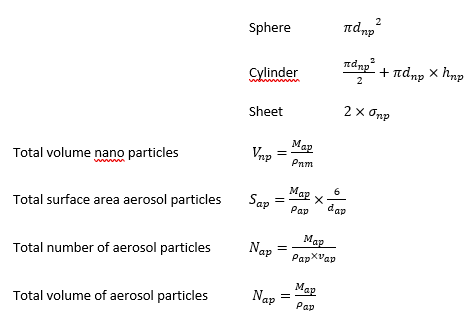
These dose metrics are calculated from the particle properties provided by the model’s user, assuming that nanoparticles are adequately represented by simple geometric shapes (e.g. spheres, cylinders).
The exposure, deposition and clearance models express the output in terms of the mass of the aerosol particles and the model evaluation does not depend on the characteristics of the nanomaterial. Consequently, the different dose metrics can be calculated from the lung load expressed as mass of the deposited aerosol particles Map (g).
The aerosol particle is assumed to consist completely of nanoparticles, which means that no other components are included.
Dose metrics are calculated as:
The surface area of the individual particle is calculated from the specification of the nanomaterial geometry by the user. ConsExpo nano supports three particle geometries: sphere, cylinder and sheet. For these, the surface areas are calculated as:
These calculations are based on the single valued (i.e. median) aerosol diameter and single valued nano material particle size.
When distributions of aerosol particle and nano particle diameters are considered, calculations change.
Following inhalation and deposition in the alveoli, the nanomaterial may dissolve and be removed. Dissolution may occur in the lung-lining fluid, or after phagocytosis in the alveolar macrophages. ConsExpo nano accounts for slow dissolution in the alveoli. Dissolution is modeled using a user-specified dissolution rate k (specified as a fraction of deposited mass dissolved per unit of time).
The deposited mass Mdep will change in time due to dissolution according to a linear kinetics decay as:
Mdep(t)=Mdep0 x e-k×t
Dose metrics other than the mass will be evaluated by assuming that the primary nanomaterial will not change due to dissolution, but only the diameter of the deposited aerosol will shrink according to
d=d0e-k/3×t
The various dose metrics are then re-evaluated by first calculating how many nanoparticles will fit in the aerosol particle of reduced size, and then repeating the dose calculations for the different dose metrics with adjusted total mass and aerosol size distribution.
In risk assessment, exposure is to be compared with some level of hazard level, usually a level that is considered ‘safe’. Such a hazard level is oftentimes derived from inhalation studies in rat and is usually expressed in terms of an amount that is inhaled (e.g. may be inhaled daily per kilogram body weight without expected detrimental consequences). For purposes of risk assessment it may be informative to compare doses from a hazard study in animals in terms of the alveloar load, as this is expected to be the most relevant end point for many materials.
To this end, ConsExpo nano enables the simulation of the alveolar load in hazard study conducted in the rat. The user may specify the dosing (air concentration, number of days of exposure, duration of the daily exposure) in combination with the material (aerosol size, nano material’s shape size and density). ConsExpo nano then estimates the alveolar load combining the estimated inhaled amount with a deposition and clearance model.
Deposition in the rat
The deposition fraction of the inhaled aerosol should be specified by the user. To estimate deposition fractions, the use can be made of the Multi-Pathway Particle Deposition (MPPD) model. Clearance of material from the rat alveoli is modelled using a clearance model proposed in the MPPD model, based on data from a study by Bermudez et al., (2004). In this model, clearance is assumed to be load dependent: it decreases with increasing load, reflecting the fact that at high loads, alveolar clearance becomes increasingly impaired.
In ConsExpo nano, clearance (removal) of deposited mass m from the alveoli, is determined by a clearance rate kcl as
dm/dt=-kcl ×m
With the (load dependent) clearance rate kd (day-1)
kd= 0.03341×exp(-1.7759 ×m0.3123)+0.00072
The alveolar load (in mass) is determined by combining the exposure events during the exposure period with the clearance. In the tool, the alveolar load is evaluated by numerically solving the equations for repeated dosing and clearance.
For the comparison of exposure with dose-response information from hazard studies, it is often convenient to express the retained alveolar dose in a specific normalisation (see for example Brown et al., (2005)) . Normalisation of the load on a physiological scale allows usually for a more meaningful comparison of doses across species (man and rat, in the case of ConsExpo nano) or for a better scaling between in vivo and in vitro systems. Normalisations that are frequently useful are: the retained dose per kg body weight, per g lung, per unit of the alveolar surface area. Less frequently used normalisations include: the retained dose per alveolus or per alveolar macrophage.
ConsExpo nano facilitates all these normalisations. The user has to provide input on specific physiology such as lung and body mass, surface area of the alveolar epithelium. ConsExpo nano provides defaults for all these parameters for both human and rat. Defaults are taken from Brown et al., (2005).
|
Normalisation |
Divide by (defaults) |
|
|
|
|
Physiological parameter |
Human |
Rat |
|
Total retained (default) |
|
|
|
|
Per kg body mass (/kg) |
Body weight |
65 (kg) |
330 (g) |
|
Per g lung mass (/g) |
Lung weight (g) |
1100 |
1.7 |
|
Per m2 alveolar area (/m2) |
Alveolar area (m2) |
57 |
0.30 |
|
Per alveolus (-) |
Number of alveoli |
5 x 108 |
2.0 x 107 |
|
Per alveolar macrophage |
Number of alveolar macrophages |
6 x 109 |
3 x 107 |
In a typical assessment, ConsExpo nano performs what are often called ‘deterministic’ evaluations: the user specifies single values for the different input parameters and ConsExpo evaluates a single number for the exposure.
In addition to this, ConsExpo nano also facilitates probabilistic evaluations. The user may specify probability distributions as parameter input. Whenever distributions have been specified for input, ConsExpo conducts a Monte Carlo simulation. Rather than calculating exposure only once, ConsExpo nano repeatedly samples the input distributions of all distributed parameters. With each sample the inhaled and deposited dose are evaluated, yielding a distribution of calculated outputs. This distribution is then presented as the outcome of the evaluation.
In sampling the distributions of input parameters, ConsExpo nano observes limits of validity on the parameters. If samples of parameter inputs are encountered with one or more parameter values outside the validity domain (e.g. negative body weight, a weight fraction larger than 1), the sample is discarded and a new sample is drawn.
ConsExpo nano supports the following distributions:
Normal
Requires the mean µ and standard deviation σ as input. The distribution is defined by the probability density function:
Log Normal
Requires the median µ andgeometric standard deviation (GSD) as input. The probability density is defined as:
and
σ=log(GSD)
Uniform
Input is required on a lower bound xo and an upper bound x1 of the distribution. The probability density is defined as:
Triangular
Requires as input (a) location (minimum), (b) scale (maximum) and (c) shape (maximal probability). The probability density varies between the minimum and maximum values and has maximum probability at the ‘shape’. It is defined as:
Beta
The Beta distribution can be used to specify probabilities between 0 and 1. Specification requires input on two positive shape parameters α and β.
For guidance on the use of the Beta-distribution see for example Information Technology Laboratory (2012).
Bermudez, E., J. B. Mangum, B. A. Wong, B. Asgharian, P. M. Hext, D. B. Warheit and J. I. Everitt (2004). "Pulmonary responses of mice, rats, and hamsters to subchronic inhalation of ultrafine titanium dioxide particles." Toxicological Sciences 77(2): 347-357.
Hedwig M Braakhuis, Margriet VDZ Park, Ilse Gosens, Wim H De Jong and Flemming R Cassee (2014). “Physicochemical characteristics of nanomaterials that affect pulmonary inflammation”. Particle and Fibre Toxicology, 11:18
Delmaar, J.E., M.V.D.Z. Park, J.G.M. van Engelen (2005). “ConsExpo, Consumer Exposure and Uptake Models. Program Manuel”. Bilthoven, The Netherlands: National Institute for Public Health and the Environment (RIVM). RIVM Report no. 320104004
Delmaar JE, Bremmer HJ (2010). “The ConsExpo spray model - Modelling and experimental validation of the inhalation exposure of consumers to aerosols from spray cans and trigger sprays”. Bilthoven, The Netherlands: National Institute for Public Health and the Environment (RIVM). RIVM Report 320104005
ICRP 66, (1994). Human Respiratory Tract Model for Radiological Protection.
ICRP, (2015). Occupational Intakes of Radionuclides: Part 1. ICRP Publication 130. Ann. ICRP 44(2).
MPPD model. https://www.ara.com. Accessed 30-09-2019.
Morrow PE. (1988). Possible Mechanisms to Explain Dust Overloading of the Lungs. Fund. Appl. Tox. 10: 369-384.
National Institute of Standards and Technology Information Technology Laboratory (2012). "NIST/SEMATECH e-Handbook of Statistical Methods 1.3.6.6.17. Beta Distribution". April 2012. Accessed 16/10/2019.